A lab technician wearing a full body protection suit inspects a bottle containing growth media for virus production during coronavirus vaccine research at the Valneva SA laboratories in Vienna, Austria. (Photo: Akos Stiller/Bloomberg via Getty Images)

South Africa’s first Covid-19 vaccines are on course to touch down at OR Tambo international airport on Monday, but they won’t carry the security measures that could best protect them from criminals.
The one million AstraZeneca shots, which have been produced by and procured via the Serum Institute of India, will be given to frontline healthcare workers, so the vials are set to be transported to some public health facilities at which the theft of medicines has been prevalent.
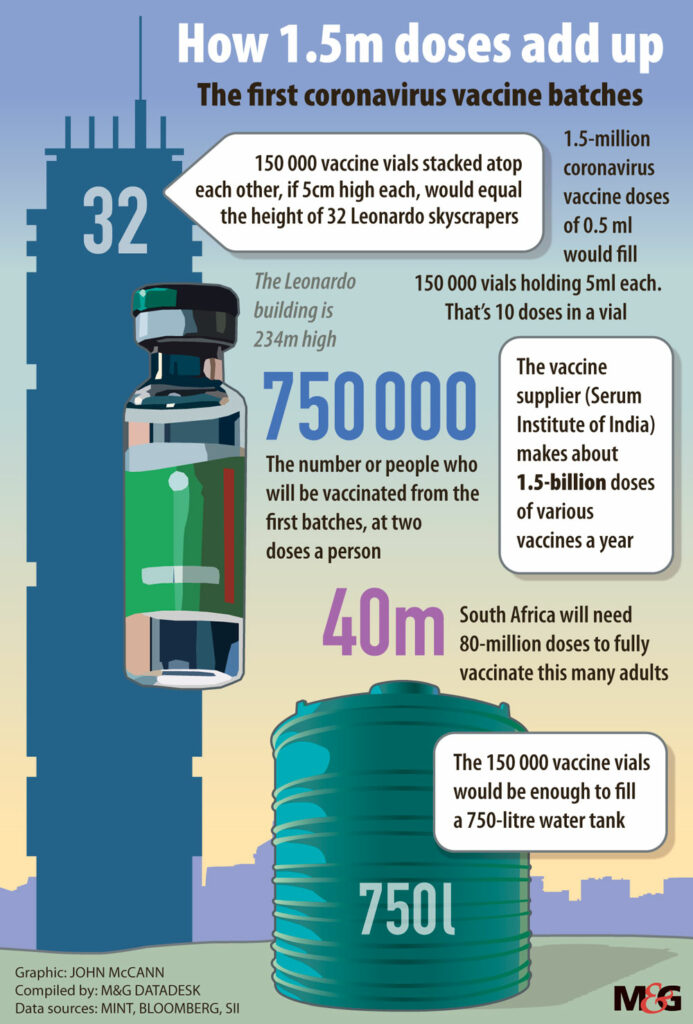
The Serum Institute, as well as the health department, have confirmed to Bhekisisa that the vaccines will not be barcoded at all.
A source at the largest producer of vaccines in the world said: “The government of India compels us as a manufacturer to use serialisation-and-traceability barcodes, but in these times exceptions are being made; shipments have to be rushed.”
Earlier this month, deputy director general of health, Anban Pillay, said all vaccines entering the country would be barcoded to prevent falsification and theft.
The health department also announced last night that it would track vaccines by means of an electronic vaccination data system (EVDS) on which everyone receiving vaccines would have to register.
On its website, Gavi (the global vaccine alliance), which heads up the international procurement mechanism, Covax, through which South Africa will be procuring most of its jabs, explains the EVDS that the country will use is as follows: “The EVDS would provide and track vaccine information (type administered and batch number); patient information, including demographics and number of doses; safety information (possible adverse events following immunisation); and details of vaccine administration sites.”
During a health department briefing on vaccines on 3 January, Pillay said: “We have learnt from the distribution in other countries that the safety and theft of vaccines is a problem, so we will be certainly tracking the vaccines and the vehicles moving them through.
https://bhekisisa.org/resources/2021-01-05-when-can-south-africans-expect-a-covid-19-vaccine-and-how-will-it-be-delivered/
“There’ll be a track and trace of vaccines using barcode scanning, as well as the safe and secure disposal of these packaging, vials and data verification linked to the volumes that have been submitted,” said Pillay
Our vaccines won’t be constantly visible in the supply chain
Rob Botha, a healthcare logistics and technical expert in the health department, who will be co-ordinating South Africa’s Covid-19 vaccine supply chain, confirmed that the first vaccines would not arrive with barcodes on them, but, “even if it [the vaccine] doesn’t have a barcode on it, we will still be tracking it using the batch [number] and the expiry date of the product. You’ll be able to link the product directly to the patient.”
For the past seven years, Botha has managed two USAID-funded projects aimed at strengthening the country’s public-sector medicine supply chain.
He says “full track and traceability” of Covid-19 jabs would not happen during South Africa’s vaccine rollout, because the health department had not yet adopted the “overarching” system that would make this possible.
The system Botha is referring to has been developed by GS1, an international not-for-profit organisation that specialises in supply chain security. The system has been approved by the World Health Organisation (WHO) and World Customs Union to mark vaccines with GTIN (global trade item number) 2D datamatrix barcodes.
The technology allows for visibility of medicines throughout the supply chain, from point of manufacture until a patient is given a medicine.
GS1 Africa healthcare manager, Nuran Idris, says the system enables medicine barcodes to be scanned at “every point” in the supply chain. If the scan doesn’t happen at any point, the system “flags” this, and investigations begin immediately.
In many cases, she says, falsified and substandard medications never reach their destinations, because investigations and subsequent action by the authorities “remove them from the equation” and, if theft happens, it’s detected “very shortly thereafter”.
Idris, who is based in Nairobi, says a scan of a datamatrix barcode linked to the GS1 system also offers access to an international database of medicines and medicine supply chains, enabling checking and counterchecking of product information.
“Authorities can identify shipments wherever they are in the supply chain, and there’s assurance that the right products reach the right people because there’s end-to-end visibility,” she says.
Idris says other security systems register medicines at point A, and then again at point F, but because they’re not recorded at other points in the supply chain, “there are times when they disappear from the radar”.
‘We are far more vulnerable than we even know.’
Botha expects most future vaccine shipments to contain the datamatrix barcodes and he says while it’s not possible for the health department to use them to their “full potential”, they will be used to “good effect”, even if it’s just for verification.
“In public sector clinics, for example, they’re using what’s called the stock visibility system, or SVS. It has barcode-scanning functionality to identify the product, if the user wishes to use that.”
But Andy Gray, senior lecturer in the pharmacological discipline at the University of KwaZulu-Natal’s School of Health Sciences, says the use of barcodes at any stage during the vaccine program would be an exception to the norm, as medicines are generally not barcoded in South Africa.
“It’s truly bizarre to think that every single item in a supermarket has a barcode on it, and yet we don’t have barcodes on our medicines,” says Gray.
He is a member of the WHO expert panel on drug policies and management, as well as a member of several committees at the South African Health Products Regulatory Authority (Sahpra).
Gray says South Africa has, for decades, relied only on batch numbers stamped on packages of pharmaceutical products to keep them safe.
“These are imprinted or sort of squashed, cut into the cardboard. Both of those technologies are very easy to falsify. Those who are making falsified goods will put a batch number on it and they might even go and find a legitimate one so that the batch number is in that series.”
Even if the health department does use the datamatrix barcodes that are on vaccines, Gray says some public hospitals and clinics have “poor computerisation”, meaning that barcodes and inputting them into systems won’t always be possible.
He does, however, think the SVS, where functional, could work well to protect vaccine stock if there’s “excellent” co-ordination and communication among roleplayers.
“Nurses are sending in stock reports on the SVS on their cellphones. So there are ways of harnessing some of the capacity that we have. In our private sector, we have real-time data sharing. You go into a pharmacy: before they dispense your script, they’ve already checked with your medical scheme whether they’re going to pay for it or not,” he says.
“We’ve just got to make sure that all of the elements of that system are brought to bear on the same problem at the same time.”
But Gray is concerned that vaccines remain at risk of being stolen. “I think we are far more vulnerable than we even know. We’ve certainly had theft from the provincial [medicine] depots and we have a lot of theft [of medicines] happening from hospitals. In fact, we’ve had problems with theft on demand, where people just phone in to a member of staff and a box gets packed up for them.”
‘We don’t know how much falsified medicine there is’
Idris says South Africa could have had “complete track and tracing” and “full end-to-end visibility” of vaccines across most of its public health sector, but it never followed through with a plan to implement the GS1 system. In a notice published in the Government Gazette on 15 September 2017, the health department indicated its intention to implement the GTIN-14 datamatrix barcode requirements.
“The barcode is used for the unique identification of trade items worldwide and leverages existing global standards. The requirement seeks to harmonise with the global health marketplace to enable end-to-end data visibility, identify and implement supply-chain efficiencies, ensure supply-chain security and improve patient safety.”
Botha says the biggest driver of implementing the barcodes in most countries is to prevent falsified medicines and counterfeit pharmaceutical products from entering supply chains.
Gray says the government has not bought into the GS1 system “because it doesn’t think we had a big problem” with fake and substandard medicines. Like the rest of the world, he said, the country didn’t foresee having to regulate the rapid entry and distribution of millions of vaccines in a short space of time.
However, South Africa’s largest private hospital group, Netcare, is using the barcodes to track and trace medicines in its 54 hospitals, having forged a relationship with GS1 in 2016 and subsequently invested in the necessary technology and networks.
Gray says South Africa’s reputation is of having a secure medicine supply chain, but it’s not known how much falsified medicine there is at any given time in the country.
“We don’t know much about South Africa’s market,” says Gray. “We don’t proactively sample the market. We react to reports of quality problems and those are then usually investigated by the manufacturer, not by anyone else.”
Mlungisi Wondo, acting manager of Sahpra’s regulatory compliance unit, confirmed this and said that there are countries that have track and trace systems to eliminate counterfeits and products that they don’t want.
“Our system is still purely manual, hence we enforce compliance on companies to be responsible for their products through good manufacturing practices, or GMPs, or good vigilance practices, also commonly referred to as GVPs.”
Gray says South Africa’s reliance on “good manufacturing practices” to keep fake medicines out of the country could mean “it’s missing problems that are occurring. So, if our medicines go across into neighbouring countries, is somebody slipping falsified versions into those countries? We don’t know. Are some of the importers’ medicines that are arriving on our shelves not the ones that we expect to find? We haven’t detected any, but it’s not impossible that they are happening.”
This is the first in a four part series on COVID-19 and organised crime from by the Bhekisisa Centre for Health Journalism. Sign up for the newsletter. This investigation was made possible with a grant from the Global Initiative Against Transnational Organised Crime (GI-TOC).
