We danced that night: Funiwe with photographs of her with her late twin sister Funeka at their matric ball in 2008.
It was May 23 2010 and 21-year-old Funiwe Mbhamhama woke to the beautiful but forlorn sound of church singing. She looked at the sun streaming in through the crack in the orange curtains of her Khayelitsha home. It wasn't Sunday. There should be no singing.
She had a sinking feeling as she pulled back her powder-blue duvet. As she walked out of her bedroom, she heard the sounds of people praying in her house. She only had to look her mother in the eyes to know what had happened and she burst uncontrollably into tears. Her twin sister Funeka had died in the night after a two-year battle with an extremely drug-resistant form of tuberculosis.
"She went to my mother's room just before midnight and fell on the bed. Then my mother realised she was not breathing. I hurt a lot because she was not just my sister, she was my twin," Funiwe said.
During her matric year in 2008, Funeka contracted extensively drug-resistant tuberculosis, known as XDR-TB, which is resistant to four of the major drugs used to treat TB. Funiwe said that Funeka was sent home from the Brooklyn Chest Hospital in Cape Town about a month before she died. "They told her there was nothing they could do because the treatment wasn't working."
Just three years later, Funiwe developed the same illness as her sister and is facing the same battle – the one her sister had lost.
One in ten XDR patients are cured
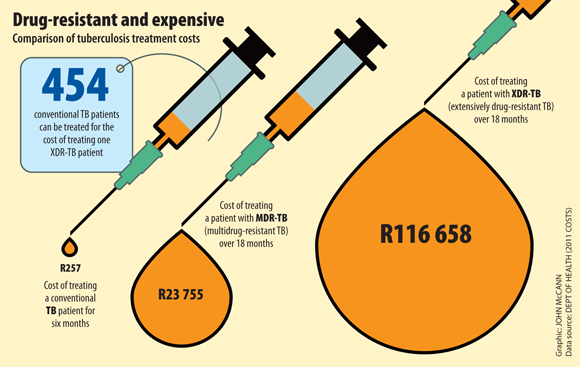
According to a study published in the January edition of medical journal the Lancet, only 11% of South Africans with XDR-TB between 2008 and 2012 were cured – 73% died. The treatment, the study says, takes between 18 and 24 months – but it often fails to cure patients because the XDR-TB bacterium has become resistant to most available drugs.
Resistance can develop when antibiotics are not taken correctly or TB treatments are stopped before the end of a drug course.
But such drug-resistant strains of TB can then be transmitted from one person to another, according to the World Health Organisation (WHO). Department of health statistics show that more than half of drug-resistant TB cases in South Africa are acquired rather than as a result of a patient taking their treatment incorrectly.
But new treatments are becoming available to treat even XDR-TB. Since December 2012, a few of South Africa's estimated 1 500 annual XDR-TB patients have had access to a new unregistered drug, bedaquiline, through a controlled programme that is being run like a clinical trial. To date, just over 40 people with XDR-TB have used the drug.
"It's a new medicine with a novel mode of action: it attacks the ability of the germ that causes TB to produce energy," said Francesca Conradie, the president of the South African HIV Clinicians' Society and drug resistant TB clinical advisor for Right to Care. "It's a miracle drug by all accounts; it makes patients become noninfectius much sooner than other available drugs."
For her twin it was too late, but Funiwe is one of the fortunate ones. Almost a year after starting on bedaquiline, she has gained back much of the weight she lost, about 13kg, since being diagnosed with XDR-TB and is feeling much better. "My mother was thinking I will also pass away like my twin sister. I thought so too."
Clinical access programme
Although bedaquiline has been granted accelerated approval by the United States Food and Drug Administration, it is not freely available in South Africa because clinical trials have not yet been concluded. They are necessary in order to apply to the Medicines Control Council (MCC) to register a drug for use in the country.
The first two phases of trials, the safety and early efficacy phases have been completed for bedaquiline in a small group of about 300 people. The last phase, during which the drug needs to be tested on a considerably larger group of people and which will start later this year, is likely to have results by 2020, according to Conradie.
But, she said, patients can't wait that long.
"Janssen Pharmaceutica [the pharmaceutical company that manufactures bedaquiline] offered us a clinical access programme where they would provide the drugs under certain conditions so we approached the MCC for special approval, which was granted," Conradie said.
The programme consists of expert TB clinicians who evaluate patients on a case-by-case basis. An application is then submitted to Janssen who approves each patient before they can start taking the drug.
"Bedaquiline is like a precious diamond – we have to look after it but we can't hold it so tightly that no one has the benefit of it."
SIde effects
Conradie said there are strict criteria that a patient has to meet, including being over 18 and not pregnant, because the drug has not been tested for safety in these groups.
There are significant side effects and restrictions regarding the use of the drug and patients with some types of heart problems can't use it. Bedaquiline prolongs the QT interval in the heart, a measurement of its electrical cycle, "which can cause a fatal arrhythmia [irregular heart beat]", she said. Patients are therefore screened for heart problems and are monitored closely while using the drug.
So far the risk is theoretical, Conradie said, and there have been no reports of cardiac-related deaths in patients receiving the drug.
But even when bedaquiline has undergone the rest of the trials and if it is approved by the MCC, she hopes its use will still be strictly controlled – in other words, that clinicians won't be able to prescribe it freely in either the public or private sectors. "Indiscriminate use of this antibiotic could lead to further resistance, rendering it ineffective."
Linezolid
Another new hope in the field of drug-resistant TB is linezolid, a drug produced by the pharmaceutical company Pfizer, which was developed for the treatment of other drug-resistant superbugs, not related to TB, commonly found in hospitals, according to Julia Hill of Médicines Sans Frontières (MSF).
But, since 2006, the WHO has also recommended it for treatment of both multidrug-resistant TB (which is resistant to two of the main TB drugs) and XDR. "It's an extremely powerful antibiotic and, because TB is a bacterial infection, it treats the condition," Hill said.
Most people cannot afford it. Pfizer charges the private sector R589.10 a tablet and the state R287.90, according to MSF. Patients need a daily tablet for up to two years, which amounts to a staggering R430 000 a patient in the private health industry and R210 167 through a public health facility.
A generic version has been made by the Indian company Hetero at a cost of R25.30 a pill. But although Pfizer's patent will expire in August this year, Hetero's version still needs to registered with the MCC before it can be bought by the government. According to Hill, that can take up to four years.
Anban Pillay, head of financing at the health department, said that Hetero submitted its generic for registration to the MCC last year. The department has since asked the MCC to fast-track the process, which can be done when medicines are needed urgently.
"This practically means the application goes on top of the pile and is given to the first available reviewer to look at," Pillay said.
"The hope is that Hetero's drug is approved soon by the MCC so that, if Pfizer continues to want to supply at a price that's unaffordable, we then have a second option by the time Pfizer's patent lapses later this year," he said.
When Hetero's generic of linezolid becomes more accessible in South Africa, its use should also be closely monitored to protect the drug's efficacy, he said. (See "SA and India face same problem for different reasons")
21 pills
After a long day at university and a stop at the clinic to collect her drugs, which include both bedaquiline and linezolid, Funiwe arrives at home.
"I have to take all my pills after my university classes, around 6pm, because they make me sleepy," she says.
Dressed comfortably in a light-green tracksuit and white takkies, she sits on her bed and pours the tablets out of the ziplock packet on to the duvet. She lays out the green, orange, white and brown pills in a neat row on the bed.
She splits the 21 tablets into two groups and reaches for the glass of water on her bedside table. She gulps down 10 tablets and winces. "Those green ones are really bitter and leave a bad feeling in my throat," she says.
She takes a breath and swallows the rest, followed by another big gulp of water. "I think I will be cured because now there is treatment that works but, when Funeka had TB, there wasn't," she says, throwing the empty packet into the bin. "Take your treatment and follow the doctor's orders," she laughs. "You'll be fine."
SA and India face same problem for different reasons
The incidence, or at least the detection, of multidrug-resistant TB (MDR-TB) is increasing and, from 2011 to 2012, the greatest growth was recorded in India, South Africa and the Ukraine, according to World Health Organisation statistics.
Drug-resistant strains of TB can be transmitted from one individual to another, according to the WHO.
In South Africa, data from the department of health shows that more than half of MDR-TB patients had not contracted any other form of TB before. But, in India, experts blame the indiscriminate use of antibiotics for the increase in MDR-TB.
Patients often do not take their medication as instructed, do not complete their courses, or are given incorrect prescriptions, which can lead to the resistant form of the disease developing.
Nalini Krishnan, director of the TB organisation, Reach, based in Chennai in Tamil Nadu state, said: "Drugs intended for drug-resistant TB are routinely prescribed. You will find prescriptions for five, even six, different TB drugs for someone who has contracted normal TB for the first time," she said.
National guidelines stipulate four drugs should be prescribed.
She said TB antibiotics are also routinely prescribed for other illnesses, further complicating each individual's resistance profile.
A 2010 study published in the scientific journal Plos One, which was undertaken in India's most-populated city Mumbai, showed that only three of the 106 private practitioners surveyed could write a proper prescription for MDR-TB.
Misuse of antibiotics in India "unprecedented"
"The misuse of antibiotics in India, and South Asia in general, is absolutely unprecedented," said Leena Menghaney of India branch of Médicines Sans Frontières' (MSF).
The majority of the population would rather use the private health sector than the state's, which has a dedicated TB programme from which patients can access the correct drugs for free. This exacerbates the problem.
According to Krishnan, as much as 70% of the population turn to the private health sector first for the treatment of any condition.
"There is an inherent belief that, if you go to a government centre, the timing is inconvenient, you'll have to wait for a very long time and you probably won't be treated very well."
However, in South Africa the majority of TB patients get their treatment from the state, particularly in the case of drug-resistant TB.
"Most medical schemes don't reimburse for TB drugs, because they believe the state is providing an adequate service for TB treatment," said Anban Pillay, the head of finance at the health department.
The department is not concerned with restricting access of TB drugs in the private sector because there is so little access already. "The private sector also doesn't really have the diagnostic capabilities in terms of identifying drug-resistant TB, so the lab analysis is usually done by the state anyway … and the private sector mostly doesn't stock the drugs."
India and SA are in sharp contrast
But in India the situation is in sharp contrast.
"Even though private healthcare is often inexpensive, it's only when the money runs out that these patients find their way to a government facility and by that time diagnosis has often been very delayed," Krishnan said.
To prevent resistance developing to new TB drugs coming on to the market, such as the Belgian pharmaceutical company Janssen's new drug bedaquiline (for extensively drug-resistant TB), an expert committee for the regulation of newer TB drugs in India was formed early last year.
"We realise that allowing bedaquiline to be sold like all other antibiotics would be a mistake," said Menghaney.
"But, in India, committees like these have a track record of tending to be so bureaucratic.
"We have the same problem with HIV treatment where a special expert panel has to recommend second- or third-line treatment [for resistant strains of HIV] and patients have died waiting for the committee to make their decision," she said.
Clinical trials for bedaquiline have not yet been completed. These are usually needed before medicines are registered in a country. But in South Africa for example, some patients have access to the drug through a special programme being run like a clinical trial.
Indian patients are not as fortunate, however.
According to Menghaney, the MSF has submitted a recommendation to the TB medicines regulation committee for a similar programme as South Africa's. "But, since January, we have had no response
