Johannes Phokela at his Selby
COMMENT
Mapololo walks into the clinic wearing high-heeled shoes and a big smile. Two months ago, on her first visit to the health centre, she was a shell of the woman.
Her body was wasting away and it was racked by an incessant cough. Sometimes – the scary times – she coughed up blood, “lots and lots of blood”. Before her doctor could even ask about her symptoms, Mapololo provided a diagnosis of her own – she said she had “strong TB”, a colloquial term for multidrug-resistant tuberculosis (MDR-TB).
She knew this because her boyfriend was fighting for his life in Cape Town’s Brooklyn Chest Hospital and he had shown the same symptoms.
The most striking thing about Mapololo on her first visit to the clinic was her palpable fear, not only for herself but also for her two young children.
On her most recent visit, however, she is a person transformed. She has gained weight, painted her nails and no longer coughs as much. The haunted look in her eyes has been replaced by something more vital, a look of hope.
Several factors have contributed to Mapololo’s metamorphosis: access to healthcare near her home; rapid diagnostic tests that enabled doctors to confirm their shared suspicions; and a regimen containing new drugs to treat her MDR-TB.
Serious public health problem
Three years ago, her story might have been very different. But the department of health has been nothing short of revolutionary, making sure that South Africans have access to the latest developments in the field of MDR-TB as quickly as possible.
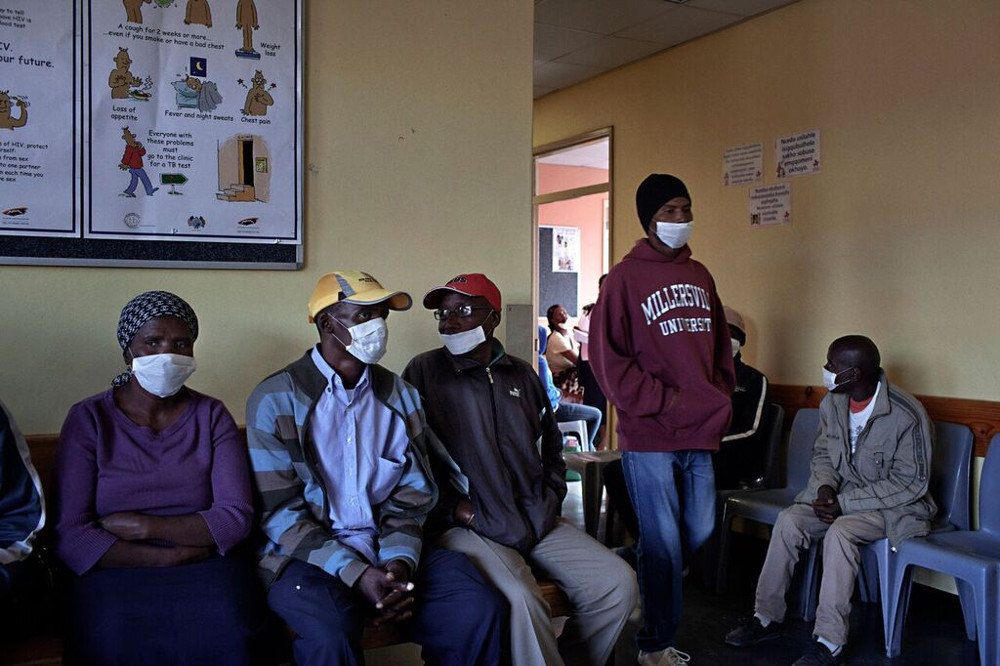
TB patients wait for treatment at an MSF clinic in Khayelitsha, Cape Town. (Photo: Supplied by MSF)
MDR-TB is one of the most serious public health problems facing the country. This type of TB does not respond to two of the drugs forming the backbone of a standard TB drug course. The National Health Laboratory Service estimates that about 4% of the country’s 380 000 TB cases are drug-resistant (DR-TB) cases, or around 15 000 people.
According to the Western Cape Health department, drugs used to treat a patient with ordinary TB cost about R350, those for an MDR patient could cost up to R50 000 — or more if new drugs are included in the regimen.
But South Africa faces additional challenges, including a 70% TB-HIV co-infection rate and extensively drug-resistant forms of TB (XDR-TB). Even fewer drugs are available to treat patients with this type of TB.
Treatment of MDR-TB is difficult and lasts for 18 to 24 months, including a daily injection, with many toxic medications.
XDR-TB is even more difficult to treat, and the five-year survival rate for XDR-TB in South Africa is 20%, lower than for most malignancies. But XDR-TB culture conversion rates, which is an early sign that the two-year treatment might ultimately be successful – have climbed to about 50% today in South Africa, a result of promising new drugs.
The country is showing the world what a strong response to fighting “strong TB” can achieve. The health department systematically provides access to new rapid diagnostics for MDR-TB, such as GeneXpert and Line Probe Assay, and to bedaquiline, the first new drug introduced for TB treatment in 50 years.
Model for new drug use
In the past six months, more than 1 100 people in South Africa have been started on treatment that includes bedaquiline, and the department is hoping to put at least 3 000 people with DR-TB a year on the drug.
South Africa has more people being treated with bedaquiline than any other country in the world, and is providing a model for showing how new drugs can be introduced in a high-burden country.
Bedaquiline is not the only reason South Africans with MDR-TB have reason to hope. Another medication called delamanid has also been shown to significantly increase the chance of a cure. The drug was first tested on South African patients in 2009 and, after showing initial efficacy here, is now registered and available for use in the European Union, Japan and South Korea.
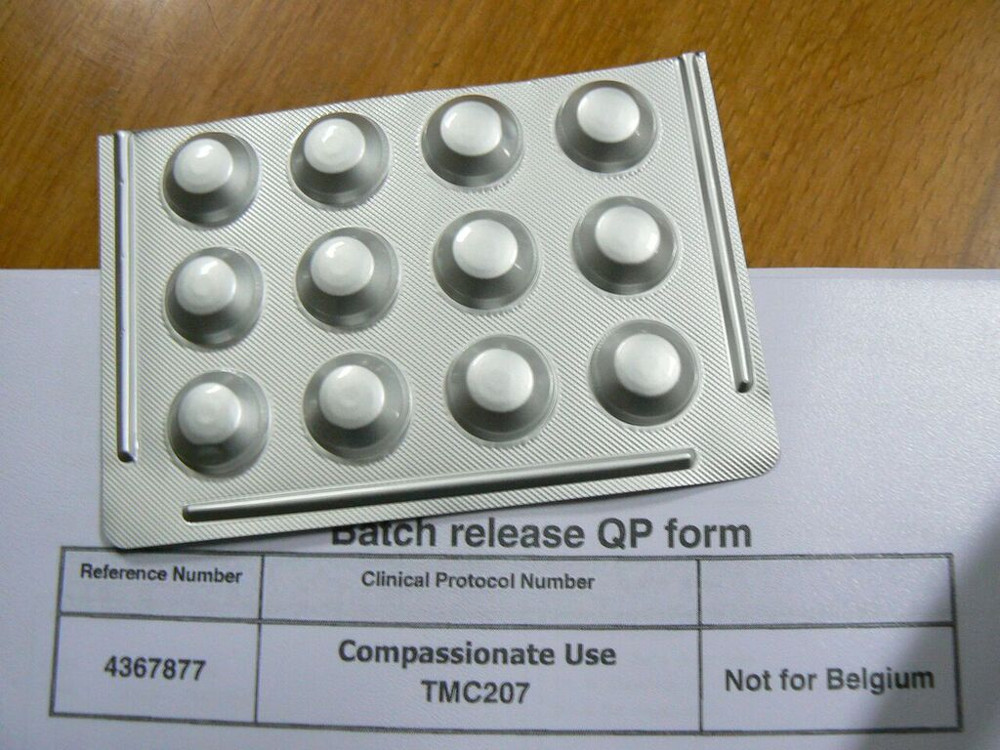
The drug, Bedaquiline, is being used to treat drug-resistant TB in South Africa. (Photo: Supplied by MSF)
About 10 patients in South Africa have access to delamanid on compassionate grounds, but estimates suggest that 7 000 people in the country would benefit from the drug if the World Health Organisation recommendations on its use were followed.
Delamanid is especially important for use in South Africa because it can be given with antiretroviral therapy (ARVs) to patients co-infected with HIV. It has also been shown to be safe and effective in children as young as six.
Otsuka, the Japanese company that manufactures delamanid, and the health department-appointed group responsible for introducing bedaquiline in the country have held discussions on how to begin providing delamanid. Early access for a select number of patients would be a positive development, but action for longer-term solutions is urgently needed.
The ethical reasons for registration
Otsuka has yet to register delamanid in South Africa, which could take more than a year. Registration would allow the nation’s MDR-TB experts to develop suitable guidelines for the use of the drug.
It would be an ethically sound decision, because Otsuka not only tested the drug on South Africans with TB, but is continuing to learn more about its optimal use here.
The global price of new drugs is a cause for concern. At at least $900 per patient for bedaquiline, and $1 700 per patient for delamanid, the financial burden of offering new treatments in combination with other expensive DR-TB drugs will be significant for high-burden countries.
But if access to better treatment is limited because of the price, it will be a serious setback for a country where activists fought for affordable generic ARVs in the early 2000s.
Bedaquiline and delamanid offer a real reason for those living with the disease – and those who care about them – to have hope for DR-TB treatments that offer greater chances of success.
Mapololo’s face falls a little when she talks about her children.
If they should get sick with the “strong TB”, they will need delamanid, and she has heard it will be hard to get it here.
She wants to make sure her boys have the same chance she did to benefit from a medication that got much of its start in South Africa.
She wants them to get the best treatment possible, and have a reason to hope too.
Dr Jennifer Hughes runs Doctors Without Borders’ DR-TB treatment programme in Khayelitsha, Cape Town