An unknown man has been shot and killed at an Eastern Cape initiation school.
The health department plans to have "formal talks" with traditional leaders about the possibility of introducing a new nonsurgical device to circumcision ceremonies, according to national HIV director Thobile Mbengashe.
Mbengashe told the Mail & Guardian that the health minister had made it clear that he "would like to ensure that traditional circumcisions become safer and we would like to look into the feasibility of training traditional circumcisers on how to use the PrePex device", which was approved by the World Health Organisation (WHO) in May.
Last week Parliament held a "snap debate" on the death of 35 initiates in one month – 29 in Mpumalanga and six in Limpopo – because of botched circumcisions. According to ANC chief whip Mathole Motshekga, who addressed the national assembly, the deaths were a "painful wake-up call for us to embark on a holistic and national transformation" of initiation schools," he said. "These schools cannot be allowed to become slaughter houses."
Motshekga quoted a 2010 report by the cultural, religious and linguistic rights commission, which said that 145 boys died between 2009 and 2010 because of complications related to their circumcisions, and another 1?200 were admitted to hospital.
According to the chief whip, these are "alarming statistics and despite the practice having survived many generations, we may have to re–examine the ways in which it is carried out in this modern age".
This may require "stringent health interventions", he said.
Respect our tradition
The chairperson of the Congress of Traditional Leaders of South Africa, Kgosi Setlamorago Thobejane, said his body would welcome talks with the government if the "ideas are presented in a way that respects our African tradition. We welcome proposals that can play a positive role in our initiation ceremonies but wecan't allow anything to undermine our cultural practices. A medical practice can't simply substitute our tradition," he emphasised.
Mbengashe said his department has "no intention of undoing traditions" and would "work in partnership" with traditional groups.
"We are conscious of the fact that PrePex may not be acceptable to traditional circumcisers, which is why we intend to respectfully engage with them and come up with a joint solution," he said.
Traditional circumcisers rarely have a medical background, but use a knife or spear to cut off all or part of the foreskin of the penis. Wounds sometimes become septic due to a lack of infection control or knives not being sterilised properly between procedures.
Tzameret Fuerst, the president of the Israeli company Circ MedTech, which manufactures PrePex, said: "Now is the time to find a safe and simple solution for nonmedical practitioners to perform circumcisions. Traditional circumcisers can be trained quickly on how to use PrePex and conduct the procedure safely in an unsterile environment as part of their wider traditional practices around the right of passage into manhood."
Circumcision with PrePex
PrePex is the first nonsurgical device that has been endorsed, or "prequalified" by the WHO. The scientific prequalification process required Circ MedTech to run six major independent PrePex studies that were audited on site by the WHO in at least two countries. The process was completed last month.
The device uses an elastic band that compresses the foreskin against a rigid plastic ring that is placed on the inside of the penis's foreskin. The elastic band cuts off the blood supply to the foreskin, which loses sensation and dries out, similar to the process of removing the umbilical cord of a newborn. The PrePex has to be worn for a week, after which it is removed and the dead foreskin is cut off.
According to Fuerst: "It's a bloodless procedure that does not require the use of injected anaesthetics or the cutting of live tissue and can be performed by health workers with a lower skill set than those required for surgical procedures."
While doctors are needed for medical circumcisions, the prequalification trials proved that "low cadre" nurses can administer PrePex … and because no live tissue needs to be cut, the three–minute placement and removal procedures don't have to be performed in sterile environments such as theatres.
"Anyone who successfully passes the three–day PrePex certification training programme can be a PrePex operator. Technically, you do not need to be a medical practitioner. However, it's up to the government of a particular country to set the policy," Fuerst said. "The procedure has been studied in the hands of low cadre nurses, therefore a bridging trial would be important to validate safety and efficacy in the hands of traditional practitioners."
The plastic device was tested on men in Rwanda, Zimbabwe and Uganda as part of the countries' medical male circumcision campaigns, which are different from traditional male circumcision. A traditional circumcision is a rite of passage to manhood, and the foreskin–cutting procedure forms part of a larger tradition that involves spending several days in the mountains or bush. Medical circumcision campaigns, however, involve a surgical procedure aimed at reducing HIV infections among men, in which the entire foreskin is removed. In traditional ceremonies, often only part of the foreskin is removed.

Medical male circumcision in SA
The South African health department began medical male circumcision efforts in April 2010 after it was proven that the complete removal of the penis's foreskin reduces a heterosexual man's chances of contracting HIV by 60%.
After the medical circumcisions started, a study in Orange Farm outside Johannesburg showed that this preventive benefit increases to 76% after three years.
According to the health department's deputy director general for male circumcision, Dayanund Loykissoonlal, 910?330 South African men had been medically circumcised by the end of March 2013 – 329?000 of those had been carried out by nongovernmental organisations supported by the United States government's HIV fund, Pepfar; the rest by the health department.
The government's national strategic plan on HIV for 2012 to 2016 aims to have medically circumcised 80% of men between 15 and 49 – or 4.3 million men – by the end of the 2015/2016 financial year. The cumulative cost for this (from April 2010, when the medical circumcision campaign started) is R2.1 billion.
Modelling studies published in the medical journal PLos Med in 2011 showed that if South Africa reaches this target, more than 20% of new HIV infections – or one million – could be averted by 2025. According to study projections, about five medical male circumcisions are needed to prevent a single case of HIV infection.
Mbengashe said his department is behind on its targets and "unlikely to achieve its goals if additional modalities that can help to scale up the medical circumcision process are not introduced. The government is keen to investigate the use of PrePex as part of its medical circumcision efforts, as well as an option for traditional circumcision, as it allows for procedures to be performed almost anywhere, doesn't routinely require doctors and is quicker to perform than surgical procedures," he said.
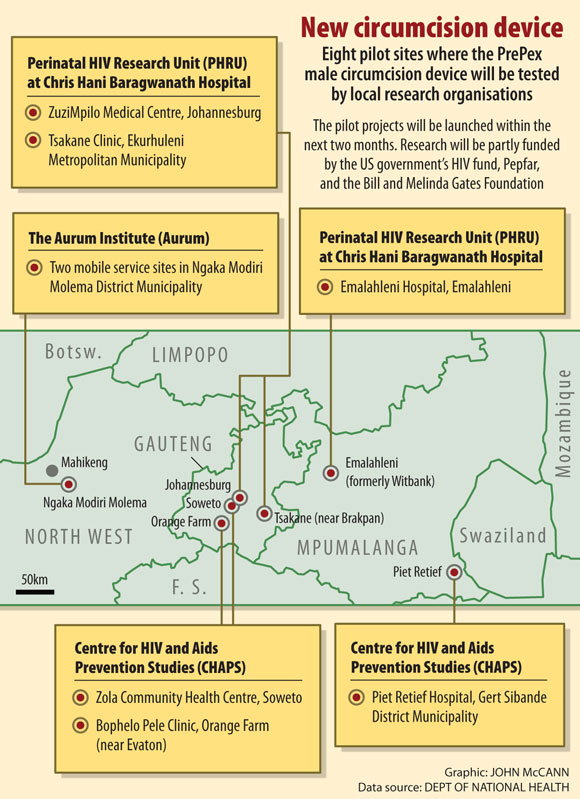
Within the next two months, the health department will be launching eight PrePex pilot sites in Gauteng, Mpumalanga and North West to test the feasibility of introducing the device as part of its medical circumcision campaign – in addition to the option of surgical circumcisions.
According to Mbengashe, the state will also be sending a delegation to Rwanda to learn from PrePex practitioners. The country was the first in Africa to introduce the device and has established a "centre of excellence" around the device.
Cost: a stumbling block
However, cost remains a stumbling block to introducing PrePex. While some studies have shown that circumcisions with the device work out cheaper than surgical ones, many experts are concerned that, at its current price of R200 per device, its use won't be sustainable in the developing world. Unitaid, which finances the WHO prequalification programme, has warned that "more market competition is needed" to make PrePex "affordable for supply to the world's poorest". Executive director Denis Broun cited a recent study in Uganda in a press release thatfound the cost–effectiveness of PrePex to be only "2% less than the current surgical method, because of its high price".
According to the WHO website two more nonsurgical devices – the Shang Ring from China and the Turkish AlisKlamp – are also going through the prequalification process. Julia Samuelson from the WHO HIV prevention department said the Shang Ring, which is the furthest along the process, is expected to receive prequalification before the end of the year.
Fuerst said her company is in price discussions with Pepfar and the Global Fund to Fight Aids, Tuberculosis and Malaria – which are expected to fund the purchasing of PrePex devices in African countries once local pilot studies have been concluded satisfactorily.
"We're all trying to achieve the same goal and it is a priority to find a price that works for the fast, broad distribution of PrePex. I'm confident that price will not be an obstacle to the scale–up of this life–saving intervention in Africa," she said.
According to Dino Rech from the Centre for HIV and Aids Prevention Studies in Johannesburg an obstacle to using PrePex as a traditional circumcision device is that the tool has only been prequalified for circumcisions performed on men 18 years and older. The centre's Orange Farm Bophele Pele project was the first in South Africa to conduct medical male circumcisions with the aim of reducing HIV infections and also works with traditional circumcisers in the area to maketraditional circumcision procedures safer.
"At least half of our clients, who are often from cultural groups that traditionally circumcise, are below 18, which means that we won't be able to use the PrePex on them," he said.
According to Sema Sgaier from The Bill and Melinda Gates Foundation, this situation could change. The foundation is supporting trials in Zimbabwe and Rwanda that aretesting the device on groups of boys aged 13 to 17 and 10 to 17 respectively.
Bloodshed and pain
Rech said the Centre for HIV and Aids Prevention Studies has worked with three groups of traditional circumcisers with whom they've negotiated to allow doctors to perform the traditional circumcision procedure surgically at the clinic.
"Traditional circumcisers normally have two clear requirements: there has to be bloodshed and pain. In our consultations with them, we normally settle on a local anaesthetic injection as qualifying for pain and the blood loss during surgery takes care of the blood shedding.
"With the PrePex, however, there is no needle involved and no blood. That may be a problem to traditional circumcisers," he said.
According to Rech the healing time – a period during which men can't have sex – for the PrePex is eight weeks as opposed to the six weeks required for surgical procedures.
"We already battle to get our clients to adhere to the six weeks, so eight might be a challenge. We also need to see what men would do during the seven days thatthey have to wear the device and what happens if it gets displaced," he said.
Last year The National HIV Communication Survey, which was conducted by the Johns HopkinsHealth and Education South Africa, loveLife and Soul City, found that of the men intending to get circumcised in the country at the time, 80.5% preferred to be medically circumcised.
However, a study by the Desmond Tutu HIV Centre at the University of Cape Town – which was published in the South African Medical Journal in 2012 – interviewed 200 Xhosa men, most of whom had been traditionally circumcised. It revealed that while 66% of the men were aware of the preventive benefit of medical circumcision, most were unwilling to have it. They mentioned religion, culture, notions of manhood and social disapproval as reasons.
Rech said the biggest difficulty with speeding up medical male circumcision campaigns – more so than the cost or finding doctors or nurses to conduct the procedure – has been to "create the demand for the supply of it".
Mbengashe said that before 2010 South Africa had no demand for medical circumcisions.
"Yet we've managed to get almost one million men to undergo the procedure," he said. "There's much room for improvement and the PrePex can help us to create more demand for safe circumcisions, because it provides men with another option to choose from in the case of medical circumcision. And, in the case of traditional circumcision, it might provide a safe alternative for initiates to be circumcised without destroying tradition."
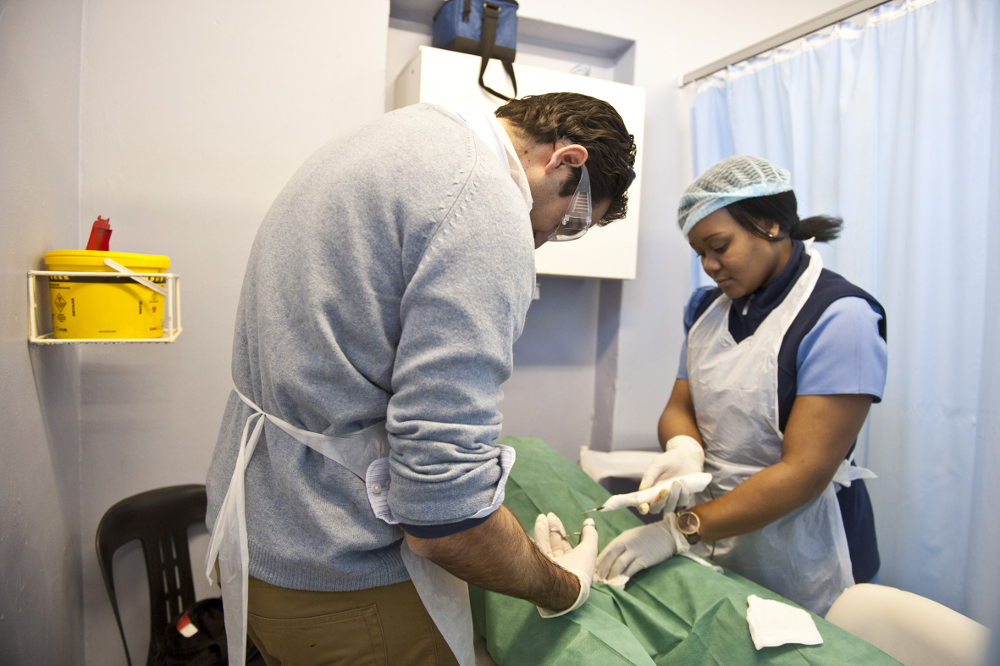
Dr Dino Rech of the Centre for HIV and Aids Prevention Studies performing a surgical circumcision at the centre's Orange Farm site (Delwyn Verasamy)
___________________________________________________________________________________
Clamp controversy still not cut and dry
Taramedic Corporation, the Malaysian company that manufacturers the controversial Tara Klamp, a nonsurgical male circumcision device that is used by the KwaZulu–Natal government, requested application forms from the World Health Organisation (WHO) to begin the prequalification process for the device six months ago, according to Julia Samuelson from the WHO HIV–prevention department.
Prequalification is a stringent scientific process conducted by the WHO before endorsing a medical device. In the case of nonsurgical male circumcision devices, it requires a manufacturing company to run several independent trials, audited on site by the WHO, in at least two countries to prove that the tool is safe and efficient.
"There is, however, not yet an indication that the company is going to proceed with the prequalification process as we have not heard back from them. They have notsubmitted any application forms," Samuelson said.
The Tara Klamp was mired in controversy when the provincial government started using it aspart of its medical male circumcision campaign – without WHO endorsement and when no scientific data testifying to its safety and efficacy had been published in medical journals.
The HIV advocacy organisation, Treatment Action Campaign, considers the Tara Klamp to be "unsafe, more expensive and only marginally faster to use than standard methods of circumcision" in an analysis of the device on its website. The Centre for HIV and Aids Prevention (Chaps) conducted a study on the TaraKLamp in Orange Farm near Johannesburg in 2011 and in its report published in the South African Medical Journal it cautioned against its use because some research participants experienced bleeding, infection and problems urinating.
According to the national health department's HIV director, Thobile Mbengashe, the KwaZulu–Natal government is running Tara Klamp trials and the procedures are "well controlled and documented".
The Mail & Guardian reported in 2011 that Zulu King Goodwill Zwelithini received a R1–million luxury sports utility vehicle from the Tara Klamp's local distributor, Tariq Yusuf, after the king gave the provincial health department the go–ahead to roll out the clamps, which were procured from Yusuf's company. However, Yusuf maintained that the gift was a "token of respect" which came "from the bottom of my heart".
Zwelithini explicitly endorsed the Tara Klamp in a speech to the provincial legislature in 2011.
In May, several news media reported that the KwaZulu–Natal government and Zwelithini publicly declared that no other device would be used in the province's male circumcision drive.
On May 31, the WHO prequalified another nonsurgical male circumcision device, PrePex, which was tested in Rwanda, Zimbabwe and Uganda. According to health deputy director general Dayanund Loykissoonlal, the national health department will launch eight PrePex pilot sites in the next two months and will use the device in its circumcision campaigns if the tests are successful, including in KwaZulu–Natal.
Mbengashe said those wanting a circumcision could choose to undergo a surgical procedure or use the Tara Klamp or PrePex devices. They would be informed about pros and cons of each circumcision technique.
However, Dino Rech, the medical director of Chaps, warned that the average person would not understand the difference between a device that had been prequalified by the WHO and one that had not.
"Both are plastic devices and people refer to it as iplastic. Men who have had a bad experience with the Tara Klamp may think it was the PrePex device and advise people against its use," Rech said. – Mia Malan