Desperate: Alexandra McDonald
‘Mommy!” A high-pitched scream echoed through the cold June morning in 2013. Genevieve Jorge, asleep next to her husband, slowly opened her eyes, confused.
“Help me! Mom!”
The scream, louder this time, shocked Jorge wide awake and she sat upright in the double bed. After a slight pause, while she waited for her eyes to adjust to the darkness, she struggled out from under the duvet and stumbled towards the sound of her 26-year-old daughter’s voice.
She walked in the direction of her youngest child’s bedroom but, hearing a shuffling sound to her left, she realised Alexandra McDonald was in the bathroom. As she switched on the light her eyes glanced at the clock hanging on the passage wall – just after two in the morning – before pushing open the door. Her hands flew to her face and she gasped.
McDonald looked like a child curled up on the tiled floor – her pyjamas soiled by vomit. She had collapsed and the pain in her head was unbearable.
“I don’t want to die. I don’t want to die. I don’t want to die,” McDonald repeatedly muttered, her eyes closed and her right cheek pressed against the cold floor.
Jorge recovered from her initial shock and reverted to her role as nurse and paramedic. She woke her husband, comforted her daughter and they drove her to the Donald Gordon Medical Centre in Johannesburg. By 4am McDonald was lying in a hospital bed, hooked up to medical equipment and finally pain-free as the strong drugs pumped into her arm through a drip.
Haggard and exhausted, Jorge sat next to her daughter and prayed – something she has been doing for the past few years as McDonald falls ill again and again.
Although she didn’t know it at the time, McDonald’s collapse on June 12 2013 was the beginning of a stroke – the first of four she would suffer in the coming months.
Misdiagnosis
‘Alexandra was never really a sickly child,” Jorge explains, perched on a black leather couch in the lounge of the family’s home in Edenvale, east of Johannesburg.
“But from 2009 it was illness after illness and every time they tried to help her, operating on her or giving her drugs, she survived but never got better; she just got worse and worse.”
McDonald had been incorrectly diagnosed with gastritis as well as endometriosis – for which she received an unnecessary operation.
Blood clots in her liver damaged the organ so much she had to have an emergency transplant in early 2013. But still doctors failed to find out what was wrong with her.But in January 2014, after five years, the guessing finally came to an end when, after a lumbar puncture, she was diagnosed with an extremely rare blood disease called paroxysmal nocturnal haemoglobinuria (PNH).
McDonald and Jorge had never heard of this before and neither had most of her doctors. The condition affects one to two people in a million globally, according to the United States-based medical research and treatment organisation Mayo Clinic.
There is no specific data for South Africa but Kelly du Plessis from the Rare Disease Society of South Africa, an advocacy organisation, says they are aware of only two patients in the country who have been diagnosed with PNH – McDonald and a man in his 30s who lives in Durban.
According to the Mayo Clinic, the red blood cells of PNH patients break down too soon, causing a variety of symptoms, which differ from person to person and include uncontrolled blood-clotting, abdominal pain, anaemia and kidney disease. On average, people survive for 10 years after being diagnosed but “some patients can survive for decades with only minor symptoms”.
Treatment out of reach
A new drug developed specifically for the condition is changing the outcome for PNH patients – but only those who can afford it.
Called eculizumab, branded Soliris by Alexion Pharmaceuticals, the US company that makes it, the drug is reputed to be the most expensive medicine on the market. It costs the British government £340 000 (R7-million) to treat one patient for a year, according to that country’s National Institute for Health and Care Excellence.
Soliris is not available in South Africa – not only is it too expensive for the public health sector but medical aids are also not willing to pay for it. Even if they were, it would be a problem – Soliris has not yet been registered with the Medicines Control Council (MCC). The only way in which South Africans can get the drug, if they can afford it, is to import it directly with special permission from the health department’s director general.
According to Mayo Clinic, this injectable drug does not cure the disease but helps reduce the destruction of red blood cells so the body can function normally. But for patients to stay well they have to take the drug for the rest of their lives.
New research, published this year in the journal Clinical Advances in Hematology and Oncology, shows that PNH patients taking Soliris can lead a healthy life and live into old age. But the prohibitive cost denies many this opportunity.
 Before being diagnosed with a rare blood disorder Alexandra McDonald led a normal life
Before being diagnosed with a rare blood disorder Alexandra McDonald led a normal life
Cost of life
As soon as McDonald was diagnosed with PNH almost two years ago, Jorge has been doing everything she can to get the medicine for her daughter. “It’s been an incredibly frustrating battle because no one wants to hear us,” says Jorge.
McDonald’s medical aid, Liberty Medical Scheme, refused to fund the drug. When Jorge lodged a complaint with the Council for Medical Schemes in November last year, the insurance company won the right not to provide the medicine based on the extreme cost and the fact that the drug is not registered with the MCC.
“We can import it through a local pharmaceutical company for R80 000 per vial and Alex would need four vials for the first month and then two vials per month for the rest of her life. Who can afford that?” asks Jorge.
Soliris is registered in 50 countries, none of which are in Africa and, according to pharmacist Andy Gray, a researcher, analyst and lecturer at the University of KwaZulu-Natal (UKZN), the company can’t be forced to do so.
“There is no way for the health department or the MCC to compel any company to register any product – that is a commercial decision for the company to make,” he says.
The situation is further complicated because Alexion “has no presence in South Africa”, says Gray. “The owner of the patent has to enter into an agreement with a locally registered manufacturer or importer to even make an application to the MCC.”
He says the pricing for some new drugs like Soliris “bear little relation to the cost of production or even to the entire research and development process”, which is one of the reasons Alexion has given for the high cost.
But, says Julia Hill of the local branch of Médecins Sans Frontières, Alexion has the power to continue to sell the medicine at this “outrageous price” because it is the only drug for PNH on the market.
She adds that another justification for the high price of Soliris is that PNH is a rare illness, and therefore very few patients around the world need the medicine.
“Unfortunately, countries are seeing many newer medications or treatments for rare conditions priced well beyond the reach of many governments and any but the most wealthy individuals,” she says.
Hill argues that in situations like this pharmaceutical companies “can pull the wool over our eyes” because they are not obliged to be transparent about how they set the prices for drugs. “If information about the true cost of development was in the public domain the public might realise how much profit companies actually make.”
Hill says that one way to get access to expensive drugs is through compulsory licensing. “This means that Alexion could be forced to give another company the rights to produce the product under certain conditions and the new producer would pay Alexion back from royalties on sales.”
The new company would “likely charge a lower price closer to the cost of manufacture”. But compulsory licences are easier to issue on drugs that other companies have already started to make and when there is a need for large quantity of medicine to be produced and sold – which is not the case with Soliris, she says.
“Governments or generic producers in other countries have used compulsory licences to ensure adequate production, or greater affordability of medicines. However, this has never been successfully attempted in South Africa, in part because it is a high court process that can be time-consuming and expensive.”
Compulsory licensing
Compulsory licensing made international headlines in the late 1990s when the country’s HIV epidemic escalated but newly developed medicines on patent were too expensive for most citizens. At the time antiretroviral medication would have cost a South African a minimum of $1 000 (R13 400) a month, according to a 2005 Harvard University report.
When the government publicly opposed the high prices and amended the South African Medicines and Related Substances Act to allow compulsory licensing, US pharmaceutical companies retaliated and opposed the new legislation in court. But international pressure and the public relations nightmare led to the companies dropping the court challenge in 2001 and lowering their prices for the South African government.
Hill says this is one of the main reasons South Africa’s intellectual property policies need to be reformed.
The department of trade and industry published a draft reformed intellectual property policy in 2012 that raised concern in various sectors.
According to intellectual property academic Yousuf Vawda from UKZN, the draft policy allows for much greater ease of access to life-saving medications but “we’ve been waiting for years and we are still waiting”.
Vadwa says that, in its current form, the policy has a broader definition of the grounds on which a compulsory licence can be granted but, in the case of Soliris and other medicines that don’t have generic counterparts, this will not help patients to access the drugs.
Only once another pharmaceutical company produces a similar drug would it be possible to implement a process for price reduction.
Until the draft policy is finalised, he says, it will remain extremely difficult to get patented drugs – even if they are necessary to save lives.
Desperate for treatment
For McDonald, the politics of pricing don’t make sense.
“How can they not give me this medicine when they know about me? They know I’m going to die,” she says.
The only medication that suppresses the effects of PNH and helps to prevent potentially fatal blood clots is daily doses of the steroid cortisone. This has caused her to gain more than 20kg in the space of a year.
Now 28, she says she does not recognise herself when she looks in the mirror. “I have a huge round face and a massive belly like I’m pregnant,” she says, pulling at the edge of her black shirt.
Sitting across from her mother on an identical black leather couch, she places her right foot on her left knee. She places her foot back on the floor only to immediately lift both legs on to the couch. She explains that she’s constantly moving because she has restless leg syndrome, one of the complications of her strokes.
Looking up at the birthday cards neatly arranged between bouquets of flowers on the mantelpiece McDonald sighs.
“I’m still young. I want to work. I want to drive. I just want to be able to do things for myself like I used to.”
She looks down and back up at her mother. If she had Solaris she could stop taking cortisone – something she desperately wants to do. This will stop the weight gain but, more importantly, also the hospital visits every few months when her red blood cell count falls dangerously low.
“A few years ago I had a job I loved, a fiancé who I was about to marry and, basically, a life,” she says.
Her husband-to-be left her last year because of the years of illness.
“And now look at me,” she gestured to her stomach and lightly touched her swollen face.
“Who will want me when I look like this? I just want to look like and feel like me again.”
On September 17, shortly before the Mail & Guardian went to print, Genevieve Jorge phoned Bhekisisa with the news that pharmaceutical company Alexion has agreed to supply Soliris to her daughter free of charge. The company confirmed that she would receive her first dose of medication as early as next week
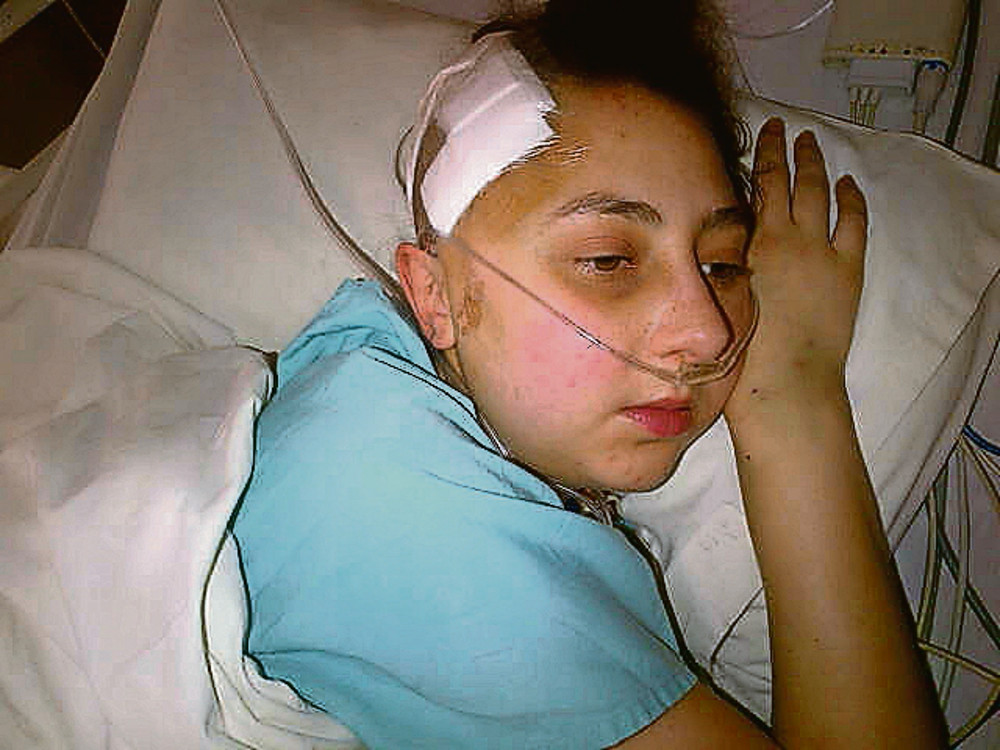 Alexandra McDonald has a rare blood disorder that has led to her having multiple strokes
Alexandra McDonald has a rare blood disorder that has led to her having multiple strokes
Orphan drugs law aims to boost production of medicine
The Orphan Drug Act, passed in 1983 in the United States, was the first piece of legislation in the world intended to increase research and development of treatments for rare diseases.
Orphan drugs, used to treat diseases that affect fewer than one in 2 000 citizens are so named “because the pharmaceutical industry has little interest under normal market conditions in developing and marketing drugs intended for only a small number of patients suffering from very rare conditions”, according to the World Health Organisation (WHO).
The Federal Drug Administration (FDA) says on its website that before this fewer than 10 orphan drugs came to market in the US. The Act allows for pharmaceutical companies to get up to 50% of their research and development costs back in tax incentives, and reduced or waived fees for FDA administration. It also allows “leniency” in clinical trials – in which the medicines are allowed to be tested for safety and efficacy on far fewer individuals than in standard trials.
Since 1983, more than 400 medicines have been given orphan drug status.
Europe followed suit almost 20 years later when the Committee for Orphan Medicinal Products, under the European Medicines Agency (EMA), was founded. It gives incentives to pharmaceutical companies similar to those in the US.
But, according to Eurodis, an organisation set up by the WHO and the EMA, it “is now clear that there will not be a tidal wave of new orphan drugs” and they estimate that only 100 new drugs will be developed from 2009 to 2019.
The organisation says this is a consequence of many governments refusing to reimburse extremely expensive drugs and because too few people need these drugs and the incentives sometimes do not mitigate the limited revenue for companies. – Amy Green
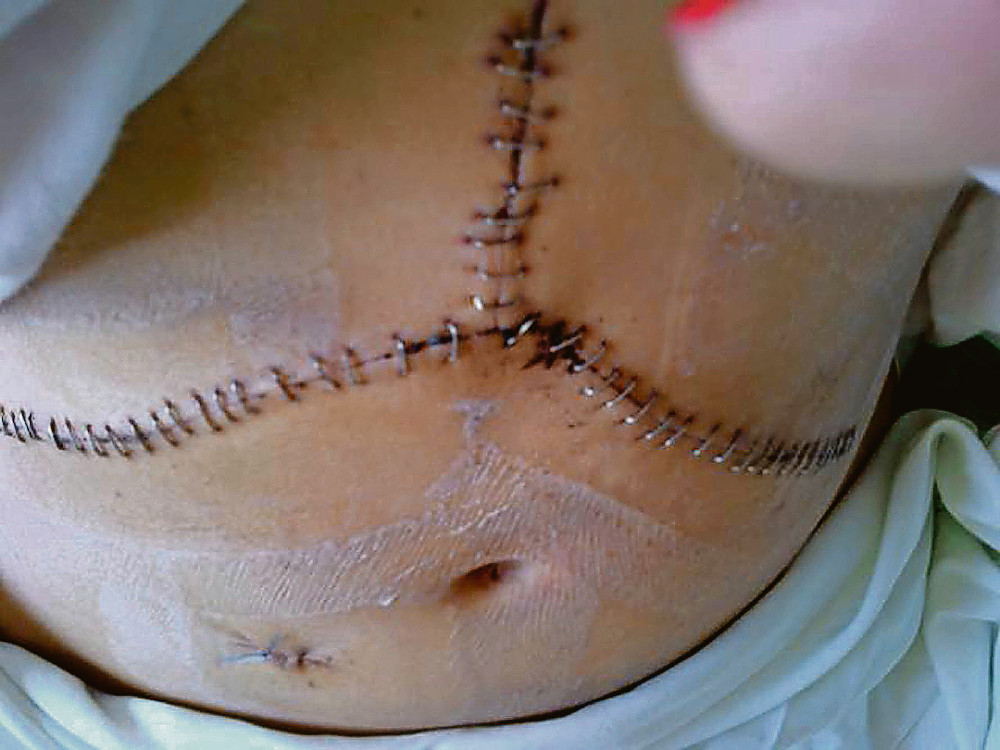 Alexandra McDonald has a rare blood disorder that resulted in a liver operation, strokes and other illnesses.
Alexandra McDonald has a rare blood disorder that resulted in a liver operation, strokes and other illnesses.
Facts about the disease
Paroxysmal nocturnal haemoglobinuria (PNH) is a rare blood disease that destroys red blood cells. The contents of these cells leak into the blood, causing a range of sometimes life-threatening health problems.
Causes: It is caused by a gene mutation in the bone marrow where red blood cells are made but it is unknown why this mutation happens.
Diagnosis: The condition can be diagnosed with a variety of blood tests. The most accurate of these is called “flow cytometry” but it is only a few years old and is not widely available.
Symptoms: Patients might live for years without being diagnosed with PNH because symptoms mimic other conditions. These include abdominal pain, severe headaches, back pain, excessive weakness, fatigue and recurrent infections. One classic symptom, which happens in half of people with PNH, is blood or dark tea-coloured urine. Thrombosis (blood clotting) is also a common symptom and, although clots can form in any vein in the body, they are often found in the liver and abdomen.
Treatment: Blood transfusions work for some PNH patients to increase red blood cell count but not for others. Medicines to help with the symptoms of PNH are usually prescribed such as anticoagulant drugs to prevent clotting. Antiplatelet medicines such as aspirin do the same.
Steroids (prednisone and cortisone) work for a few patients to slow the rate at which red blood cells are destroyed. Bone marrow transplants can cure the disease in some. In 2007 eculizamub (Solaris) was approved by the United States Federal Drug Administration as the first medicine to specifically treat PNH. – Source: Johns Hopkins University
