Milking it: The antidote to a poisonous snakebite often starts with the poison itself. Paul Rowley and Nice Casewell (above) from the Liverpool School of Tropical Medicine extract venom from a rhinoceros viper. (Photo: Nick Ballon)

Each year, millions will be bitten by venomous snakes and antivenom will remain painfully out of reach for many victims . Discovering why is a tale that stretches from the fields of West Africa to the lab and back again.
Twenty-year-old Mamadou is lying on a metal cot, eyes half-closed, breathing fast. At his bedside sits his boss, fanning him.
Mamadou is a herder. Five days ago, he was getting ready to complete his usual route when a sudden pain in his foot caught him off guard.
He had been bitten by a snake, and not just any snake: the deadly West African carpet viper.
Mamadou was first taken to a local health centre, but it couldn’t give him the care he needed. So he was then brought more than 100km to the regional hospital in Sokodé, Togo’s second-largest city.
Mamadou’s story is not uncommon. Each year up to an estimated 2.7-million people around the world are bitten by venomous snakes, the World Health Organisation (WHO) says, and about 100 000 die.
Most victims, such as Mamadou, live in poor, rural communities. Data from Africa is fragmented but a 2008 research review published in PloS Medicine estimated that snakebites kill up to 32 000 people each year in sub-Saharan Africa alone.
In 2017, the WHO finally recognised the problem by classifying venomous snakebite as a neglected tropical disease. This led to renewed discussions about the only treatment currently available — antivenom.
When properly made and administered, antivenom saves lives. But right now, the world produces less than half of what it needs. And for over 40% of the world’s snake species, there’s no antivenom whatsoever.
Even when antivenom does exist, it often doesn’t reach the people that need it.
Unstable markets have driven up costs, allowing cheaper, substandard treatments to appear. With their patchy efficacy and dangerous side-effects, these shoddy medications are deterring people in low-income countries from seeking treatment at all.
Falling demand then makes effective antivenom even more expensive.
The antivenom field is, in short, a disaster. It remains — in the words of former United Nations secretary-general Kofi Annan — “the biggest public health crisis you’ve never heard of”.
It’s warm where the snakes live. The air in the herpetarium is humid, and on the walls, faded posters sum up the history of antivenom production. The morning is coming to an end and, in neatly piled transparent boxes, 163 snakes — spanning 49 different species — are waiting to be fed.
These reptiles, housed here at the Centre for Snakebite Research & Interventions at the Liverpool School of Tropical Medicine, make up the largest and most diverse collection of venomous snakes in the United Kingdom. It’s their job to provide venom for antivenom manufacturers and to help find new ways to treat snakebites.
Today, it’s the turn of the black mamba to be “milked” — that is, to have its venom extracted.
Paul Rowley is the team’s lead herpetologist, an expert in snake handling and husbandry. Slowly, he opens the box to let the mamba out.
Rowley and his assistant work together to restrain it, pinning the animal down on the table. Holding its head tightly, they massage its venom glands to extract the liquid as the snake bites onto a small circular petri dish topped with clingfilm. The whole process takes less than five minutes.
“As soon as you move the head of the snake towards a petri dish, usually they will immediately bite, and you will get venom, too,” says Nick Casewell, a research fellow at the centre. “But it is variable how much venom you get on a particular day.”
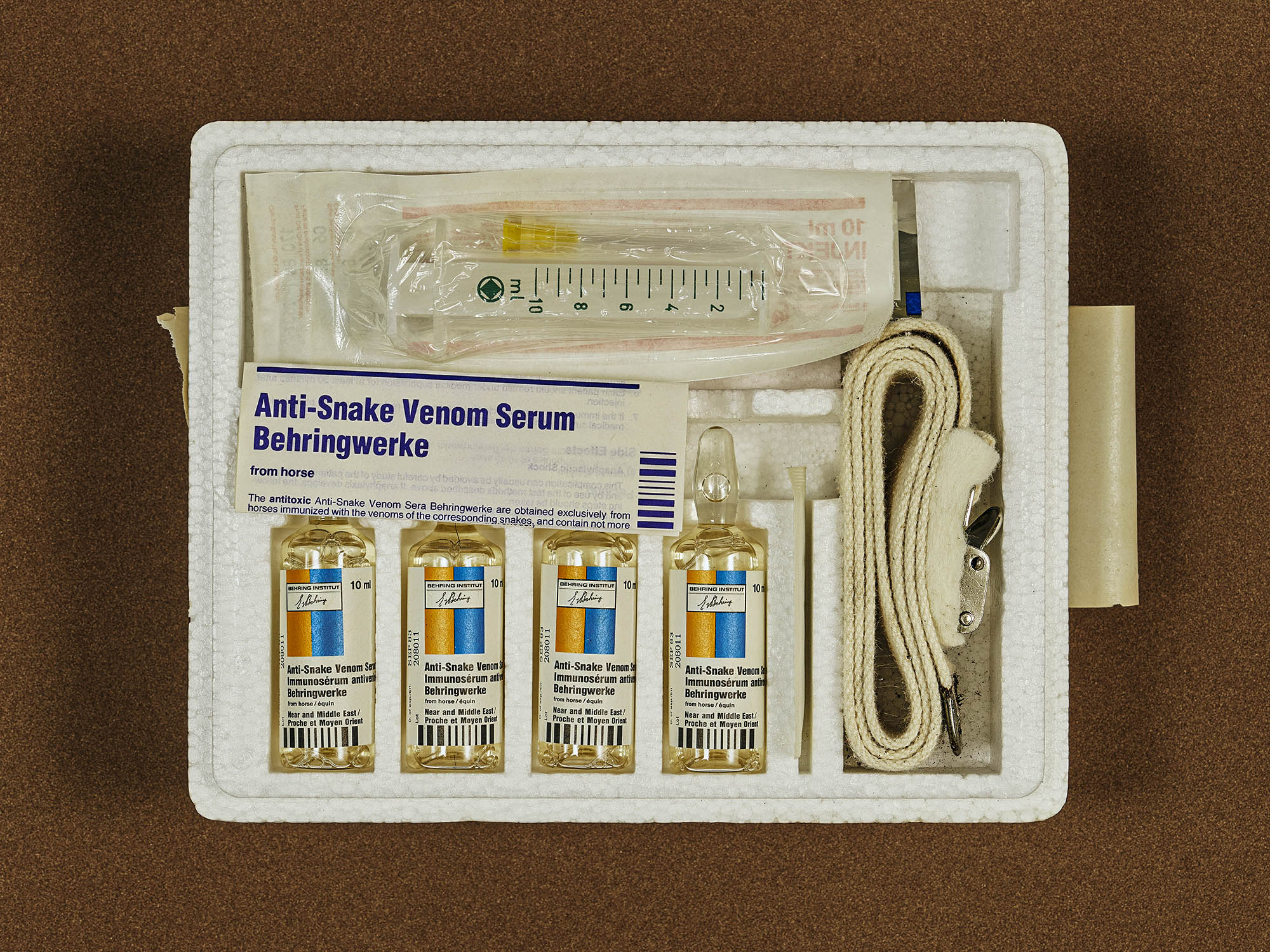
Milking a snake is the first step in creating antivenom. The process is over 120 years old, and has changed very little in that time.
Next, non-toxic doses of venom are injected into an animal — usually a horse or a sheep — to stimulate an immune response. The animal then starts producing antibodies against the venom’s toxins. Scientists will take a blood sample and from it, isolate and purify these antibodies, making them into a stable solution that can be given to patients as an injection.
This may sound simple, but it isn’t.
Because antivenom is made up of animal antibodies and foreign proteins, it can cause side effects in humans from rashes to anaphylactic shock in rare cases — especially if you don’t purify it well.
Currently, many antivenoms are made by injecting the poisons into animals like horses or sheet and extracting and purifying the antibodies they make in response for use in humans. (Photo: Nick Ballon)
Venoms are also complicated substances to treat. They’re made up of hundreds of different toxins that aren’t entirely understood. The combinations of toxins and their effects vary widely from species to species.
Broadly, there are two main families of venomous snakes: vipers and elapid snakes like the black mamba. Depending on the type of snake, the toxins in the venom cause different problems.
Fall victim to a mamba and you’re likely to sustain nervous system damage. The venom of a viper, like the one that bit Mamadou, is more typically associated with bleeding and swelling. Untreated bites like these can result in tissue at the bite site beginning to die.
Even within one type of venom, distinct toxins can create different kinds of problems. This diversity means that developing effective antivenom is difficult.
An antivenom made using venoms extracted from snakes in one place won’t work when used in another — and yet many find their way into the wrong markets.
Indian antivenom made using local species is commonly found throughout Africa, for example, despite having no effect here.
And even if the right antivenom is available, matching it to a particular snakebite is difficult.
“In at least half of cases, the snake is not seen by the patient,” says Achille Massougbodji, president of the African Society of Venomology.
But antivenom can be manufactured to work against more than one type of snake — by immunising horses or sheep with the venom of multiple species from a region. But this “polyspecific” antivenom comes with limitations.
In all antivenom, relatively few of the antibodies produced by the sheep or horse are actually specific to the venom’s toxins — studies suggest somewhere between 5% and 36%, according to 2017 research published in the journal PLoS Neglected Tropical Diseases.
In polyspecific antivenom, this figure is even lower.
“You get a mixture of antibodies that are directed towards different venoms and toxins, and you are only ever bitten by one snake,” Casewell explains. “This means only a small proportion of the antibodies in that product are actually [designed to work] against the snake you were bitten by.”
The effect, he says, is that doctors administering a polyspecific antivenom have to use higher doses, making treatment more costly and raising the risk of side-effects.
Concerns about side-effects can leave doctors wary of administering more than one vial of antivenom.
For doctors, finding the balance can be difficult without proper training. Give too much antivenom and you risk side-effects. Give too little — or too late — and it’s not going to work.
In Togo, not far from the Burkina Faso border, green fields of yams stretch for miles. The rainy season is approaching and dozens of men are already at work picking tubers.
In this area, most people work in agriculture, and they are among the most exposed to snakebite. They’re also some of the least able to afford treatment. In Togo, people have to pay for healthcare out of their own pocket.
When it comes to snakebite, the country at least fares better than some — the government has recently subsidised antivenom. And a Spanish company has also donated 8 000 vials of antivenom, which people will be able to get for free over the next year. But patients still have to pay for their hospital stay and for other medicines they might need.
On a Saturday evening in Dankpen hospital, nurse Amandine Nassimarty is the only person on duty. She is looking after 25-year-old Michel, who was bitten by a carpet viper five days ago while harvesting yams.

Camouflaged: The West African carpet viper is nocturnal and grows on average to a length of 30cm. Its venom can be deadly without treatment and can cause severe tissue damage. (Photo: Clement Carbillet/Biosphoto)
Michel has stopped bleeding, but he is still weak. Yet his relatives are asking the hospital to let him go. When they brought him in, he was unable to speak or swallow due to swelling and bleeding from the mouth, even days after the bite. But there had been no antivenom left to give him.
They had to travel by motorbike to the nearest town to buy two doses, costing 37 000 francs (R955) — or roughly one month’s salary at minimum wage — each.
“His parents are now asking to leave,” Nassimarty says. “Patients understand they should stay but they can’t pay, and often the next day I arrive and they are gone.”
Togo isn’t the only country where this happens. The price of antivenom may vary depending on the product, but it’s usually too high for families in low-income countries. In India, it’s been reported that treating a snakebite can cost up to 350 000 rupees (R75 800), when on average a farmer earned just over 8 000 rupees (R1 730) a month in 2015/16.
When someone gets bitten, family members often have to sell some of their most precious possessions — including cattle and tools — to fund the long journey to hospital and the treatment.
And the cost doesn’t always end there. Snakebite survivors — who are often the main providers for their families — may not be able to work afterwards. A family that pays for antivenom can end up facing economic ruin and so may avoid treatments in the future.
“Having a rural cattle herder or farmer lose a foot, they might survive, but they become a burden to their families,” warns Nick Brown, medical director of MicroPharm, a British antivenom manufacturer.
It’s a vicious cycle.
When some people seek out snakebite treatment, they can’t afford it, can’t find it or end up being treated incorrectly — either because the medication was poor or because it was administered in the wrong way.
This leads people to stop seeking treatment. Governments then stop buying it, reducing demand and forcing manufacturers to raise their prices. This, in turn, leads to cheaper, less safe, less effective products flooding the market, which manufacturers of good quality treatments then have to compete with — or get out of the antivenom business altogether.
This happened infamously with Fav-Afrique, one of Africa’s best polyspecific antivenoms. Its manufacturer, Sanofi Pasteur, couldn’t compete with the cost of rival products. Sanofi released its last batch in 2014 and the medication expired in June 2016. Only in January 2018 — 18 months after that final batch had expired — did another producer, MicroPharm, begin production.
At the root of this lies the biggest — and arguably hardest — problem that needs solving: the overall lack of trust in antivenom therapy.
In Liverpool, Casewell and his team are working to design safer, more efficient types of antivenom. The team has broken down the venoms of 22 of the most important snake species from sub-Saharan Africa. They’re trying to understand why venom composition is so diverse, and how and why it differs between related species.
At the Technical University of Denmark, associate professor Andreas Hougaard Laustsen and colleagues — along with the Liverpool group — are also one of the few teams investigating a new type of antivenom made up of mixtures of human antibodies grown in a lab, called monoclonal antibodies.
Generating cocktails of human antibodies is an idea that’s currently attracting a lot of interest. These types of lab-grown antibodies can target specific proteins, as in some cancer treatments, or — in the case of snakebite — specific venom toxins, clearing them out of the body more efficiently than traditional antivenom. They should also be safer to use than animal antibodies.
In 2018, a Danish team was the first to successfully use these genetically engineered proteins against snake venom toxins, in a study published in Nature Communications. Eventually, Danish scientists ended up with a cocktail of three such antibodies. When they tested it against the black mamba’s venom in mice, the cocktail stopped the venom’s effects.
Elsewhere, Matt Lewin and his team at Ophirex, an American pharma start-up, are investigating a small molecule called varespladib. It works by attaching itself to a set of enzymes that are a major common component of snake venom, and prevents them from sparking the paralysis, bleeding and muscle destruction they usually cause.
Because varespladib molecules are so small, they can work against venom in bodily tissues that antivenom can’t get to. The team has had some success using the drug to reverse snakebite symptoms and is now planning clinical trials.
All this innovative research, though promising, will take time.
But, in the meantime, if we could ensure that the antivenom that we already have is produced widely and well, that alone could be lifesaving.

From snake to lab: Venom samples are stored in a French laboratory for use in developing antidotes. To combat a lack of high-quality antivenoms in the Global South, the WHO has begun quality control checks on products. (Photo: Balint Porneczi/Bloomberg via Getty Images)
To help, the WHO is currently testing antivenom quality worldwide as part of its “prequalification” programme. Countries around the world without the national capacity to regulate medicines often rely on WHO prequalification to let them know that a product is safe to roll out nationally.
“I think that prequalification exercise will be a game-changer,” MicroPharm’s chief executive Ian Cameron says. “It will give African governments confidence that they are buying products with a minimum standard, which will drive the other products which are part of the problem out of the market.”
Mamadou never made it home from Sokodé regional hospital. He most likely died from internal bleeding caused by the venom.
The time it took for Mamadou to receive care, and the complications he suffered are a stark reminder that antivenom alone won’t be enough to solve the problem of snakebite — better health systems are needed too.
The WHO’s May 2019 snakebite strategy acknowledges this, warning that safe, effective affordable treatments will only work if weak health systems get stronger and communities seek care quickly after bites.
But this is possible.
Amavi was coming back from the market near her home in northwestern Togo when she suddenly felt the intense pain of a bite.
Thinking it was a scorpion sting — painful but rarely deadly — she decided to get some sleep and wait for the next day before seeking help.
But in the night, she started bleeding from the mouth and from a wound on her leg that she’d had beforehand. Her family realised it must be a bite from a carpet viper.
At the local health centre the next morning there was no antivenom to be had.
Wasting no more time, she perched on the back of her father-in-law’s motorcycle and travelled to the hospital, 50 km away. It took them two hours but when they arrived, doctors reacted quickly. It saved her life.
*Some names have been changed.
Full disclosure: Wellcome, the publisher of Mosaic, is funding work to transform how snakebite treatments are researched and delivered as one of its priorities. Nick Casewell currently receives funding from Wellcome.
This is an edited version of an article first published by Wellcome on mosaicscience.com. It was edited by the Bhekisisa Centre for Health Journalism and is republished here under a Creative Commons licence. Sign up to the Mosaic newsletter.