This CRPM facility was used to develop and manufacture high quality FMP2 Clinician respirators to support the Free State Department of Health, highlighting the exceptional product development capabilities in the Free State province
The vital role that Additive Manufacturing (AM) has to play in medical product development had already become clear when the Central University of Technology (CUT) developed pre-operative models of a patient’s skull to assist surgeons to plan complicated surgery down to the finest detail. This shortened the operating time considerably, which reduced the chances of complications due to prolonged operations, such as infections or excessive blood loss.
The university is proudly taking a lead in innovations that will change the face of medical science in Africa. From 2015 to date, more than 1 000 patients were assisted through the support of state and private hospitals, the expertise of the Centre for Rapid Prototyping and Manufacturing (CRPM) and funding from partners such as The Carl and Emily Fuchs Foundation and the Department of Science and Innovation (DSI).
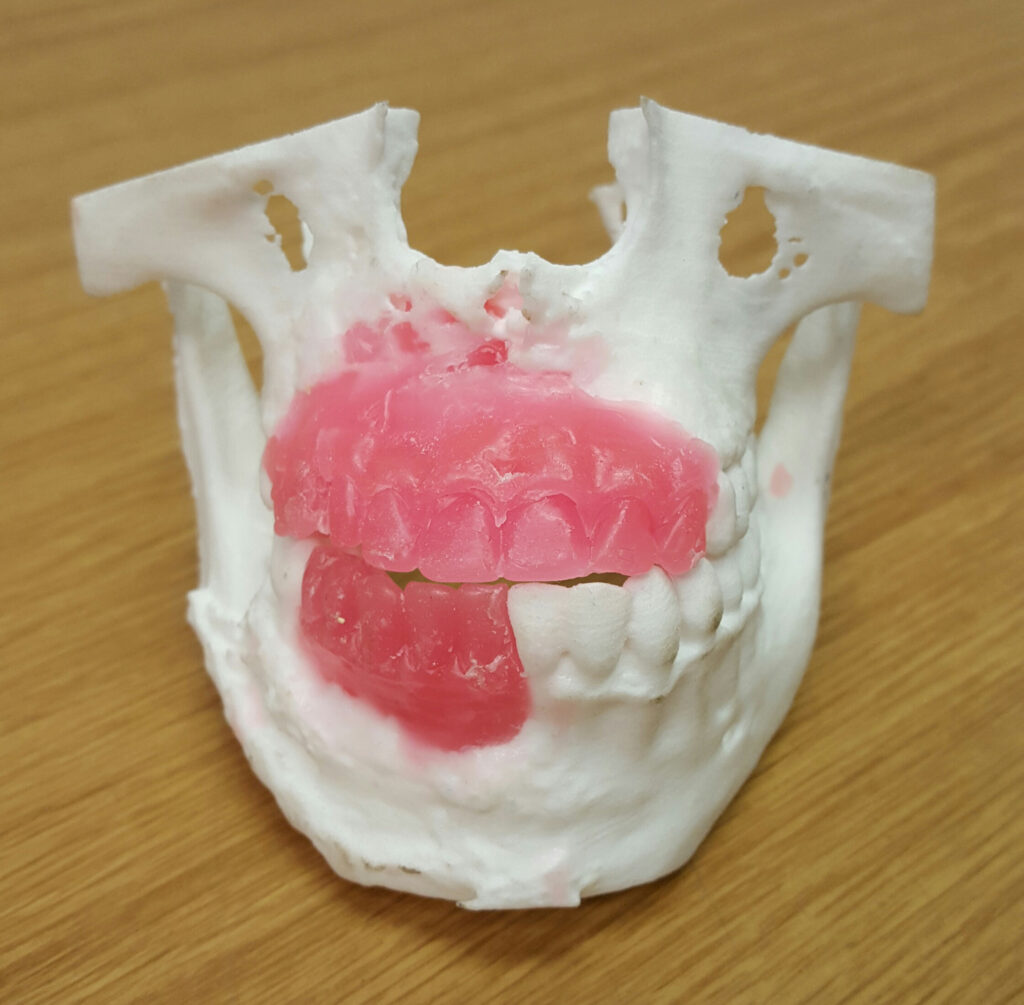 A wax reconstruction of a maxillofacial defect caused by a gunshot
A wax reconstruction of a maxillofacial defect caused by a gunshot
Some of these medical devices were first of their kinds in the country; CUT stands proudly at the forefront of innovation in this field.
“Through this technology, the CRPM’s capabilities in the design and manufacturing of patient-specific implants have undergone significant strides. Its goal is to expand its wealth of knowledge and research while forming deeper alliances with partners within business and industry, government, medical professionals, as well as private and public hospitals,” said Professor Alfred Ngowi, acting Vice-Chancellor and Principal at CUT.
The current work done by CRPM is part of CUT’s social responsibility and community engagement programme to provide a platform for the development of social and technological innovations for the benefit of society at large. The focus is to work closely with business and industry, government and community organisations to transfer its social and technological innovations that help to solve societal problems, not only for the benefit of this region, but also the rest of South Africa, Africa and beyond.
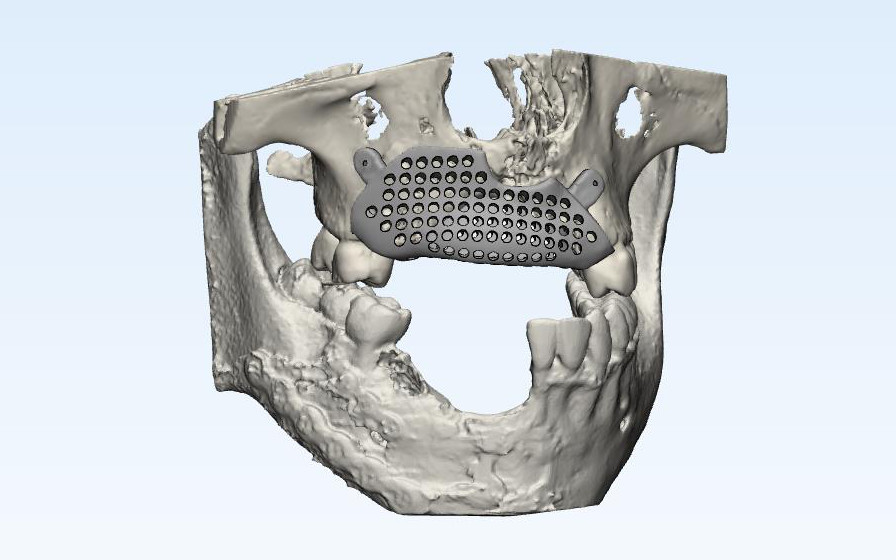 A digital rendering of a proposed prosthesis to reconstruct a gunshot defect
A digital rendering of a proposed prosthesis to reconstruct a gunshot defect
Additive Manufacturing
AM has undergone impressive growth for some time now and one of the leading centres in AM development is the CRPM at CUT in the Free State. The CRPM was established in 1997 as a centre for commercial work and research and has, since then, more than delivered on its remit.
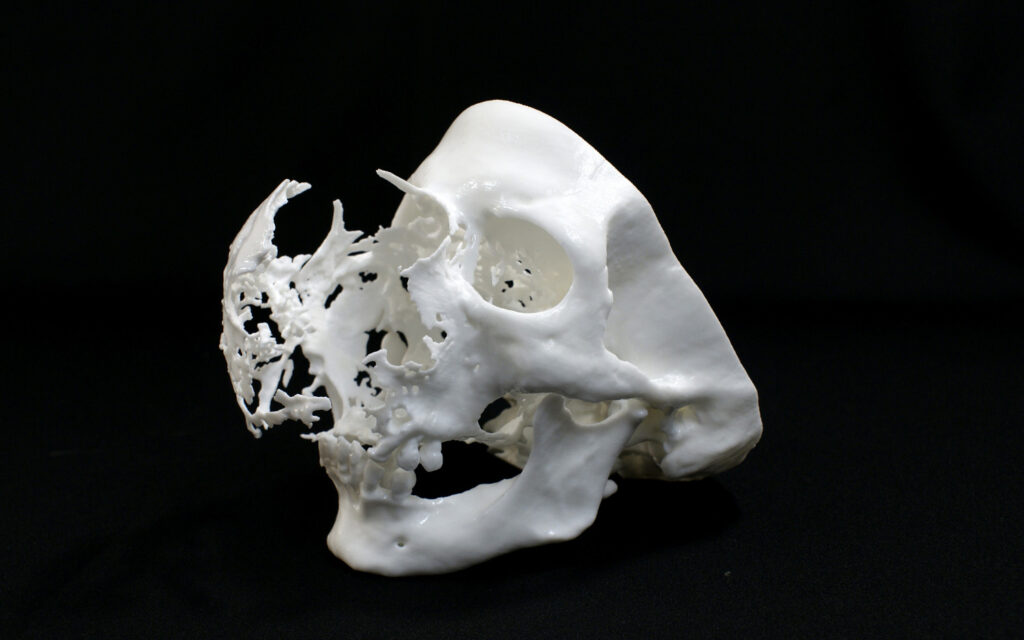 A printed planning model of a maxillofacial tumour
A printed planning model of a maxillofacial tumour
It uses rapid prototyping, rapid manufacturing, rapid tooling and medical product development technologies to further education, understanding and development. The centre has an impressive array of state-of-the-art machinery and equipment.
The CRPM received significant support and funding from the National Research Foundation, which allowed the centre to install and use the latest in technology and tools. As a result of this, the staff and students are able to maintain their lead at the forefront of research into 3D printing technology.
In 2015 CUT received an award under the South African Research Chairs Initiative (SARChI) through the DSI, as testament to the commitment that CRPM has to innovation in the AM field.
 A printed titanium prosthesis to reconstruct a maxillofacial tumour
A printed titanium prosthesis to reconstruct a maxillofacial tumour
The SARCHi Chairs are highly competitive initiatives that are tenable at universities and agencies deemed leaders in their respective fields of expertise.
This achievement has allowed CUT to proudly play a fundamental role in the new industrial revolution, and especially within the medical space.
ISO Certification
CRPM has ISO 13485 Certification for additive manufacturing of surgical planning models, cutting and drilling jigs and customised/patient-specific implants or medical devices. This makes a significant contribution to additive manufacturing in South Africa and Africa as a whole.
Due to CUT’s Additive Manufacturing research, South Africa (and the rest of the continent) is no longer reliant on Europe or America to acquire certified medical devices, which was the case in the past.
Through CRPM’s ISO certification, industrial partners collaborating with CRPM under the DSI-funded Medical Device Additive Manufacturing Technology Demonstrator (MedAdd) initiative can also focus on localisation and benefit from export opportunities.
 FS Health MEC, Motsheng Tsiu; FS Premier, Sisi Ntombela; CUT Director: CRPM, Dr Gerrie Booysen and Interim Provincial General Manager of Old Mutual, Silas Sebiloane
FS Health MEC, Motsheng Tsiu; FS Premier, Sisi Ntombela; CUT Director: CRPM, Dr Gerrie Booysen and Interim Provincial General Manager of Old Mutual, Silas Sebiloane
CUT participates in a number of strategic programmes under the Collaborative Programme in Additive Manufacturing, a DSI-funded initiative that serves as the implementation programme for the South African Additive Manufacturing Strategy. CUT currently participates in several of the subprogrammes, such as Metal Additive Manufacturing (with a focus on qualification of AM of Titanium 64 for medical implants and aerospace parts); Polymer Additive Manufacturing; Design for Additive Manufacturing; Additive Manufacturing of Platinum-Group Metals and Additive Manufacturing for Industry.
“The CRPM is growing from strength to strength and has such a fantastic line-up of achievements,” said Dr Gerrie Booysen, Director of CRPM. “We continue to find new ways of developing innovative solutions and building strategic partnerships that support our goals and ideals. The AM field is incredibly exciting right now and we are exceptionally lucky to be able to sit right at the edge of the latest developments and to take part in their evolution.”
The SARChI Research Chair under the interim leadership of Professor Deon de Beer, who is also the DSI/merSETA Chair in Innovation and Commercialisation of Additive Manufacturing, focuses on additive manufacturing of medical devices.
The medical profession is showing keen interest in the work that the CRPM and the collective AM activities (which also include the TIA-funded Product Development Technology Station) have been doing. CUT’s collective AM capabilities in the design and manufacture of patient-specific implants and other assistive devices have made impressive strides and the goal is to further this arena significantly, forming deeper alliances with medical professionals and institutions.
 Old Mutual collaborated with CUT’s CRPM to produce the much-needed clinician masks and face shields at the height of Covid-19. MerSETA CICAM, DSI MedAdd and PDTS contributed jointly towards manufacturing of injection moulding tooling so that these masks, straps and filter housing can be produced in bulk
Old Mutual collaborated with CUT’s CRPM to produce the much-needed clinician masks and face shields at the height of Covid-19. MerSETA CICAM, DSI MedAdd and PDTS contributed jointly towards manufacturing of injection moulding tooling so that these masks, straps and filter housing can be produced in bulk
“CUT’s goal is to create safe environments for medical practitioners to share their ideas and to establish a consortium between doctors and CUT’s product development centres, which will allow CUT to really drill down into new ideas and engage with what the medical community needs and ensure that CUT remains strategically aligned with real-world objectives. This led to the establishment of the DSI-funded MedAdd project at CUT,” said Professor de Beer, DSI/merSETA Chair in Innovation and Commercialisation of Additive Manufacturing at CUT.
This project aims to bridge the innovation chasm in the use of AM for the development and final manufacturing of medical devices. MedAdd enhances the current equipment and capabilities at CUT as well as enabling CUT, its academic partners and local companies to demonstrate reproducibility and scale-up of innovative medical device products.
MedAdd functions under the CRPM’s ISO certification, which acts as a “safety net” for small companies to develop and industrialise new products; this de-risks their innovative development before fully-fledged commercialisation.
In addition, MedAdd enables students, researchers, and industry personnel to develop the required skills for the development of this new technology and new industry.
 The Product Development Technology Station at CUT aims to localise medical assistive device manufacturing to reduce the importing of expensive medical equipment and devices. This project is building a sustainable and reliable solution to improve the mobility of persons with disability in rural areas
The Product Development Technology Station at CUT aims to localise medical assistive device manufacturing to reduce the importing of expensive medical equipment and devices. This project is building a sustainable and reliable solution to improve the mobility of persons with disability in rural areas
Successes achieved from developments executed during 2020 put the MedAdd project in a strong position to leverage additional funding from external partners such as NRF, GIZ, merSETA and SAiS.
As an example of strategic success that can support industry in retaining existing job opportunities or even creating new job opportunities, the PPE (personal protective equipment) developed for the Covid-19 pandemic serves as an excellent case study.
Since it was essential for companies to be listed as essential service providers to continue with production or return to work, many companies struggled to survive during the lockdown period. Due to the production, assembly, sterilisation and logistic chains created for the PPE products as developed, 139 jobs were created during the period April 2019 to March 2021.
The long- and short-term benefits to both the medical profession and the AM environment are undeniable. Through CUT’s collective AM activities and the DSI-supported MedADD, needs communicated by the medical fraternity can lead to the development and commercialisation of new products that can improve patients’ quality of life and save lives.
Add to this the long list of strategic partnerships and superb innovations, and the table is set for the collective research activities at CUT to revolutionise Additive Manufacturing in South Africa.
CUT has established itself as one of the leading institutions in Additive Manufacturing (3D Technology)
The development of a medical device industry, as well as the development of Additive Manufacturing, are key to advancing South Africa’s manufacturing industry, and CUT is making its mark in 3D Technology through the Centre for Rapid Production and Manufacturing (CRPM), which aims to stimulate the manufacturing sector in South Africa.
 The Titanium Power Bed Additive Manufacturing machine is one of the latest acquisitions at CUT; making CRPM a world class facility that specialises in Additive Manufacturing, commonly known as 3D printing
The Titanium Power Bed Additive Manufacturing machine is one of the latest acquisitions at CUT; making CRPM a world class facility that specialises in Additive Manufacturing, commonly known as 3D printing
Currently, the university’s centre is driving the Medical Device Additive Manufacturing Technology Demonstrator Project (MEDADD) at the Bloemfontein Campus, as one of the Department of Science and Innovation’s (DSI) apex projects.
This R71-million project, which is funded by the DSI, has united the university’s centre with the industry in planning and investing resources in a collaborative manner to develop locally produced medical devices through Additive Manufacturing technology, and to reduce the country’s reliance on costly imported medical devices that local hospitals cannot afford.
This is but a small contribution the university is making in this field. From a product development point of view, this is an opportunity for CUT and the government to create African solutions for South African challenges. Not only will the outcomes of this partnership improve the cost, availability and accessibility of medical devices, but will also create opportunities for economic growth in the region.